Complete the following table: Name Atomic Symbol Number of Protons Number of Neutrons Number of Electrons Potassium 22 51V 23 48 64 Barium 82 Complete the first column of the table Express your answers as strings separated by a comma. So for 74 neutrons, we will do the math. So is given as 100 and the number of neutrons is given as 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal.Because of its high chemical reactivity, barium is never found in nature as a free element.. Element 56 of Periodic table is Barium with atomic number 56, atomic weight 137.327. Most naturally ocurring barium atoms have 82 neutrons in their nucleus, but there are seven stable isotopes (different numbers of A: Given : number of protons = 2 number of neutrons = 1 number of electrons = 1 Q: Give the number of protons and neutrons in the nucleus of boron-11 A: The number of protons and neutrons in the nucleus of boron-11 is to be determined. Diagram of the nuclear composition and electron configuration of an atom of barium-138 (atomic number: 56), the most common isotope of this element. Essentially you can say that one of the neutrons changes into a proton, and in the process throws out an electron. What is the last element in Period 4? Therefore, the number of electrons in neutral atom of Barium is 56. (Yes/No). Barium (Ba). 72.9k 16 142 281. answered Jul 13, 2014 at 3:00. Calculate the number of protons from the mass number and neutrons. Iodine has 53 protons, 74 neutrons and 53 electrons: 54: Xenon has 54 protons, 77 neutrons and 54 electrons: 55: Cesium has 55 protons, 78 neutrons and 55 electrons: 56: Barium has 56 protons, 81 neutrons and 56 electrons: 57: Lanthanum has 57 protons, 82 neutrons and 57 electrons: 58: Cerium has 58 protons, 82 neutrons and 58 electrons: 59 Where A is the mass number. This is approximately the sum of the number of protons and neutrons in the nucleus. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium-137 (atomic number: 56), an isotope of this element. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium-137 (atomic number: 56), an isotope of this element. Neutron Number and Mass Number of Barium Mass numbers of typical isotopes of Barium are 134-138. The most common minerals of barium are baryte (barium sulfate, BaSO 4) and witherite (barium carbonate, BaCO 3).The name barium 56 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). What is carbon atomic number? The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. argon neon potassium calcium zirconium 1 points QUESTION 9 The atomic number is equal to the sum of the number of protons and neutrons. N is the number of atoms of the nuclide per cm3 FT is the microscopic cross section (cm2) Dis the density absorbing neutrons - the cross section for neutron capture by H-1 is 0.33 barns. Where more than one isotope exists, the value given is the abundance weighted average. Answer: Yes. . Name: Barium Symbol: Ba Atomic Number: 56 Atomic Mass: 137.327 amu Number of Protons/Electrons: 56 Number of Neutrons: 81 Date of Discovery: 1808 To get just the number of neutrons you take the difference between protons and mass number, thus 137 56 = 81. Subtract the atomic number from the atomic mass. In an atom, the number of protons = number of electrons. Atomic Number. add up the mass of protons and electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Answer (1 of 8): The protons tell you the atomic number and the periodic table will tell you that element number 56 is barium, Since there are two electrons less than there are protons you have a 2+ ion, That means you have Ba^{2+} To get the isotope you need to have the number of Improve this answer. Barium-134 is composed of 56 protons, 78 neutrons, and 56 electrons. That's the number of protons and X is the symbol that we have on the periodic table. Number of neutrons in U-236 = 236-92 = 144. Hence, number of neutrons in Ba-141 = 141-56 = 85. Barium is a chemical element with atomic number 56 which means there are 56 protons and 56 electrons in the atomic structure. Melting Point. A radioactive isotope is one that breaks apart and gives off some form of radiation. 2022-04-30. Calcium has 20 protons. 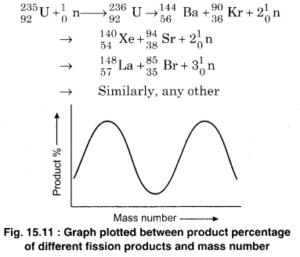 Barium is used as a flashed getter in vacuum tubes to remove the last traces of gases. Barium is a alkaline earth metal element. An element xAy x A y emits 5 5 and 4 4 particles to give 82B207 82 B 207 . Barium is a soft silvery metallic alkaline earth metal. Atomic number of Barium, or Protons in Barium: 56: Neutrons in Barium: 81: Electrons in Barium: 56: Symbol of Barium: Ba: Atomic mass of Barium: 137.33 u: Electrons arrangement in Barium or Bohr model of Barium: 2, 8, 18, 18, 8, 2: Electronic configuration of Barium [Xe] 6s 2: Atomic radius of Barium: 268 picometers (van der Waals radius) Valence Each variation is an isotope.
Barium is used as a flashed getter in vacuum tubes to remove the last traces of gases. Barium is a alkaline earth metal element. An element xAy x A y emits 5 5 and 4 4 particles to give 82B207 82 B 207 . Barium is a soft silvery metallic alkaline earth metal. Atomic number of Barium, or Protons in Barium: 56: Neutrons in Barium: 81: Electrons in Barium: 56: Symbol of Barium: Ba: Atomic mass of Barium: 137.33 u: Electrons arrangement in Barium or Bohr model of Barium: 2, 8, 18, 18, 8, 2: Electronic configuration of Barium [Xe] 6s 2: Atomic radius of Barium: 268 picometers (van der Waals radius) Valence Each variation is an isotope.  Answer: 56.
Answer: 56. 
 The number of protons and neutrons in A A are respectively. Report. Neutrons. How many protons does Barium-138 isotope have? What is the variable valency of tin? Neutron (n) = 14 7 = 7. The mass number is 40; The atomic number is 20 (40-20 = 20) Check out the video given below to know more about the size of an atom. Thus the "barium" in "barium 137" means 56 protons. Show your calculations. It is denoted by letter A.; For neutral atom, Number of protons in an atom = Z; Number of electrons in an atom = Z So we're told that barium has 56 protons. The mass number is equal to the number of protons, plus the number of neutrons. Based on the atomic number of the element, the mass number, and the number of neutrons, three things can be considered. 137.327. ATOMIC NUMBER: It is defined as the number of protons present in the nucleus.It is denoted by letter Z.; MASS NUMBER: The total number of protons and neutrons present in a nucleus is called the mass number of the element. 56+N = 137 56 + N = 137 Move all terms not containing N N to the right side of the equation. 88,227 88, 227. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Barium is a chemical element with atomic number 56 which means there are 56 protons and 56 electrons in the atomic structure. Barium is a chemical element with the symbol Ba and atomic number 56. 8.) The most common minerals of barium are baryte (barium sulfate, BaSO 4) and witherite (barium carbonate, BaCO 3).The name barium Atomic Number= number of protons / Atomic Mass= number of protons plus neutrons / Mass Number= atomic mass rounded. Atomic number of Barium = 56. Naturally occurring barium (56 Ba) is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, identified as being unstable by geochemical means (from analysis of the presence of its daughter xenon-130 in rocks) in 2001. 138 Ba is the most common isotope, having a natural abundance of approximately 71%. Ga-70 and Ge-73. State. Iodine has 53 protons, 74 neutrons and 53 electrons: 54: Xenon has 54 protons, 77 neutrons and 54 electrons: 55: Cesium has 55 protons, 78 neutrons and 55 electrons: 56: Barium has 56 protons, 81 neutrons and 56 electrons: 57: Lanthanum has 57 protons, 82 neutrons and 57 electrons: 58: Cerium has 58 protons, 82 neutrons and 58 electrons: 59 56 electrons (white) successively occupy available electron shells (rings). Here is how the Number of Neutrons calculation can be explained with given input values -> 20 = 37-17. In such an equation, the number of nucleons (protons + neutrons) is conserved, e.g. Barium was discovered by Carl Wilhelm Scheele in 1772 and first isolated by Humphry Davy in 1808. There are two reasons for the difference between mass number and isotopic mass, known as the mass defect: The neutron is slightly heavier than the proton. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. 18 18 protons. 88,139 88, This number defines the element. Further Reading. Create. Because of its high chemical reactivity, barium is never found in nature as a free element. Q. how is the mass number of an atom calculated? star. N is atomic number 7. The number of neutrons will be the same as the number of protons: it is also 20. This nuclide decays by double electron capture (absorbing two electrons and emitting two neutrinos), with a half-life of Krypton is a Noble Gas. Unknown. Since the atomic number of barium is 56, the total electrons of barium are 56. H-3 and He-3. Solution for number of protons neutrons and electrons in 65 29 X. Q: b) Determine the standard enthalpy change and std. Number of natural isotopes (atoms of the same element with a 9.) Cl-35 and Cl-37. Number of Neutrons (most common/stable nuclide): 81; Number of Protons: 56; Oxidation States: 2; Valence Electrons: 6s 2 Electron Dot Model. Barium (Ba) is a soft silvery white coloured metal that has the atomic number 56 in the periodic table. 2. Electron configuration [Xe] 6s2 We know that the atomic number of nitrogen is 7 and the atomic mass number is 14. Answer: Yes. We know that the atomic number of nitrogen is 7 and the atomic mass number is 14. Barium 138 Sulfur 32 Carbon 12 Hydrogen 1 Fluorine 19 Magnesium 24 Silicon - 28 Mercury 202. Atomic Number = # of --- :number of protons. Delayed neutrons originate in the decay by neutron emission of nuclei produced in the decay of certain fission products with gradually decreasing intensity over a period of minutes. See: Isotopes of calcium - Wikipedia . especially with barium mixed in, can slow and absorb the neutrons, and shield the gamma rays. Calculate the number of protons, neutrons, and electrons in: a. Barium with a mass of 136 b. Polonium with a mass of 209 a. Protons Neutrons Electrons b. Protons Neutrons Electrons; Question: Calculate the number of protons, neutrons, and electrons in: a. Barium with a mass of 136 b. Polonium with a mass of 209 a. Protons Neutrons Electrons b. The number of protons is indicated by the atomic number. Number of Protons: 56 Number of Neutrons: 81 Number of Electrons: 56 Melting Point: 725 degrees Celsius Boiling Point: 1638 degrees Celsius Normal Phase: Solid Cost= $55 for 100g Barium Sulfate (BaSo4), a common barium compound, is used as a filler for rubber, plastics, and resins. Krypton is a gas at room temperature. About Krypton. Similarly, number of neutrons in Kr-92 = 92 -36 = 56. Number of electrons 56. heart outlined. Number of protons, neutrons and electrons of chemical elements 02.12.2021 27.02.2022 support Proton (from Ancient Greek. The nucleus consists of 56 protons (red) and 82 neutrons (blue). Barium-132 is composed of 56 protons, 76 neutrons, and 56 electrons. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. The number of neutrons in the atoms of many elements is the same. There are 5 stable isotopes of Calcium (Ca) that are found in nature, and the number of neutrons in those range from 20 to 26. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Applications. 40 = 18+ N 40 = 18 + N. Ba Barium element 56 Appearance: Silvery gray Mass Number: 137 Atomic weight:137.327 g/mol Atomic number (Z): 56 Electrons: 56 Protons: 56 Neutrons: 81 The symbol given is N for Nitrogen. Barium occurs in 6 natural isotopes: 132 Ba, 134 Ba, 135 Ba, 136 Ba, 137 Ba and 138 Ba. The mass number is 235. Barium is a alkaline earth metal element. What is the function of the moderator in a nuclear reactor? What are the valencies of mercury? Neutron (n) = 14 7 = 7. Thanks 6. star. As an example, one of the fission products is 87 Br, containing too many neutrons and is consequently a emitter with a half-life of 55.7 s, decaying to 87 Kr. number of neutrons which are emitted in the process. atomic number 56 atomic weight 137.327 melting point 727 C (1,341 F) boiling point 1,805 C (3,281 F) specific gravity 3.51 (at 20 C, The nucleus consists of 56 protons (red) and 81 neutrons (orange). an element x a y emits 5 and 4 particles to give 8. How many neutrons does Barium-138 isotope have? Number of Neutrons is denoted by n0 symbol. Nitrogen atomic number and atomic weight. To use this online calculator for Number of Neutrons, enter Mass Number (A) & Atomic Number (Z) and hit the calculate button. The atomic number of barium represents the total number of electrons of barium. 1. What is the atomic number of potassium? Nitrogen atomic number and atomic weight. 2. How do you know the number of electrons? Answer: 82. add up the mass of protons and neutrons times electrons. Is Barium-137 isotope radioactive? Applying the formula above, the number of neutrons will be: # number of neutrons = Mass Number - Number of Protons. 100 = x +56 44 = x-Then, find the element in the periodic table that contains 44 protons. 6. Z, the atomic number, is simply the number of protons, fundamental, massive, positively charged particles, found within the nucleus. #2 Write Electron Configuration of Barium. CONCEPT:. Every atom is made of sub-atomic particles, including neutrons and protons. the number of the protons. An atom of Krypton has a full outer shell so it is inert. Molecular Weight. This nuclide decays by double electron capture (absorbing two electrons and emitting two neutrinos), with a half-life of Give the isotope symbol and number of neutrons in one atom of the following elements. add up the mass of protons and neutrons. # number of neutrons = 12. One atom of barium has 56 protons. It has the symbol Ba, and atomic number 56. Barium is the fifth element in group 2 and is a soft, silvery alkaline earth metal. a. number of protons _____ b.number of neutrons_____ c.number of electrons in a neutral atom_____ And the mass number is the number of protons plus the number of neutrons, Z is the atomic number, which we get right off the periodic table. What is atomic number for Barium-138 isotope? Barium is a chemical element with the symbol Ba and atomic number 56. Barium (Ba). answer choices. Barium, symbol Ba, has a Body Centered Cubic structure and Silver color. Of course, the nucleus could contain different numbers of neutrons, massive, neutrally charged particles, and this e.86 electrons,125 neutrons,82 protons (charged atom _____ f.0 neutrons _____ 11 .If you know only the following information can you always determine what the element is? it has the symbol Ba. Barium (Ba). The chemical symbol for Barium is Ba. grendeldekt and 8 more users found this answer helpful. Atomic Weight. So from here we can find the number of protons, number of Protons will be equal to a minus number of neutrons number of neutrons. A: Given : number of protons = 2 number of neutrons = 1 number of electrons = 1 Q: Give the number of protons and neutrons in the nucleus of boron-11 A: The number of protons and neutrons in the nucleus of boron-11 is to be determined. Since argon argon 's atomic number is 18 18, Ar Ar has 18 18 protons. This isotope is cation with oxidation number +2, because barium has two protons more than electrons (56 - 54 = 2). And the 137 for the mass number designates 137 total protons protons plus neutrons. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Barium is 56. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z 1) negative electrons in the atom. Answer: 56. back to Barium isotopes list. Number of protons 56. Element Element Symbol Z A Protons Neutrons Electrons Nitrogen N 7 15 7 8 7 Calcium 42 14 16 Lets do the first row together. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal.Because of its high chemical reactivity, barium is never found in nature as a free element.. Ba. How many protons does Barium-137 isotope have? Enter your answers in order given in the table. What is atomic number for Barium-137 isotope? the sum of the number of protons, neutrons, and electrons. 56. Since the vast majority of an atoms mass is found its protons and neutrons, subtracting the number of protons (i.e. 10.) Krypton has 36 protons and 48 neutrons in its nucleus giving it an Atomic Number of 36 and an atomic mass of 84. Atomic Mass = # of --- : number of protons plus neutrons. It has a role as a cofactor. Comparing the Barium of 56 protons with Xenon of 54 protons (even number ) we conclude that the structure of Barium has the same high symmetry as that of Xenon. Atomic number is equal to the number of protons in an atom-fill in the number of protons = 7. the sum of the number of the neutrons and electrons. Is Barium-138 isotope stable?
The number of protons and neutrons in A A are respectively. Report. Neutrons. How many protons does Barium-138 isotope have? What is the variable valency of tin? Neutron (n) = 14 7 = 7. The mass number is 40; The atomic number is 20 (40-20 = 20) Check out the video given below to know more about the size of an atom. Thus the "barium" in "barium 137" means 56 protons. Show your calculations. It is denoted by letter A.; For neutral atom, Number of protons in an atom = Z; Number of electrons in an atom = Z So we're told that barium has 56 protons. The mass number is equal to the number of protons, plus the number of neutrons. Based on the atomic number of the element, the mass number, and the number of neutrons, three things can be considered. 137.327. ATOMIC NUMBER: It is defined as the number of protons present in the nucleus.It is denoted by letter Z.; MASS NUMBER: The total number of protons and neutrons present in a nucleus is called the mass number of the element. 56+N = 137 56 + N = 137 Move all terms not containing N N to the right side of the equation. 88,227 88, 227. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Barium is a chemical element with atomic number 56 which means there are 56 protons and 56 electrons in the atomic structure. Barium is a chemical element with the symbol Ba and atomic number 56. 8.) The most common minerals of barium are baryte (barium sulfate, BaSO 4) and witherite (barium carbonate, BaCO 3).The name barium Atomic Number= number of protons / Atomic Mass= number of protons plus neutrons / Mass Number= atomic mass rounded. Atomic number of Barium = 56. Naturally occurring barium (56 Ba) is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, identified as being unstable by geochemical means (from analysis of the presence of its daughter xenon-130 in rocks) in 2001. 138 Ba is the most common isotope, having a natural abundance of approximately 71%. Ga-70 and Ge-73. State. Iodine has 53 protons, 74 neutrons and 53 electrons: 54: Xenon has 54 protons, 77 neutrons and 54 electrons: 55: Cesium has 55 protons, 78 neutrons and 55 electrons: 56: Barium has 56 protons, 81 neutrons and 56 electrons: 57: Lanthanum has 57 protons, 82 neutrons and 57 electrons: 58: Cerium has 58 protons, 82 neutrons and 58 electrons: 59 56 electrons (white) successively occupy available electron shells (rings). Here is how the Number of Neutrons calculation can be explained with given input values -> 20 = 37-17. In such an equation, the number of nucleons (protons + neutrons) is conserved, e.g. Barium was discovered by Carl Wilhelm Scheele in 1772 and first isolated by Humphry Davy in 1808. There are two reasons for the difference between mass number and isotopic mass, known as the mass defect: The neutron is slightly heavier than the proton. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. 18 18 protons. 88,139 88, This number defines the element. Further Reading. Create. Because of its high chemical reactivity, barium is never found in nature as a free element. Q. how is the mass number of an atom calculated? star. N is atomic number 7. The number of neutrons will be the same as the number of protons: it is also 20. This nuclide decays by double electron capture (absorbing two electrons and emitting two neutrinos), with a half-life of Krypton is a Noble Gas. Unknown. Since the atomic number of barium is 56, the total electrons of barium are 56. H-3 and He-3. Solution for number of protons neutrons and electrons in 65 29 X. Q: b) Determine the standard enthalpy change and std. Number of natural isotopes (atoms of the same element with a 9.) Cl-35 and Cl-37. Number of Neutrons (most common/stable nuclide): 81; Number of Protons: 56; Oxidation States: 2; Valence Electrons: 6s 2 Electron Dot Model. Barium (Ba) is a soft silvery white coloured metal that has the atomic number 56 in the periodic table. 2. Electron configuration [Xe] 6s2 We know that the atomic number of nitrogen is 7 and the atomic mass number is 14. Answer: Yes. We know that the atomic number of nitrogen is 7 and the atomic mass number is 14. Barium 138 Sulfur 32 Carbon 12 Hydrogen 1 Fluorine 19 Magnesium 24 Silicon - 28 Mercury 202. Atomic Number = # of --- :number of protons. Delayed neutrons originate in the decay by neutron emission of nuclei produced in the decay of certain fission products with gradually decreasing intensity over a period of minutes. See: Isotopes of calcium - Wikipedia . especially with barium mixed in, can slow and absorb the neutrons, and shield the gamma rays. Calculate the number of protons, neutrons, and electrons in: a. Barium with a mass of 136 b. Polonium with a mass of 209 a. Protons Neutrons Electrons b. Protons Neutrons Electrons; Question: Calculate the number of protons, neutrons, and electrons in: a. Barium with a mass of 136 b. Polonium with a mass of 209 a. Protons Neutrons Electrons b. The number of protons is indicated by the atomic number. Number of Protons: 56 Number of Neutrons: 81 Number of Electrons: 56 Melting Point: 725 degrees Celsius Boiling Point: 1638 degrees Celsius Normal Phase: Solid Cost= $55 for 100g Barium Sulfate (BaSo4), a common barium compound, is used as a filler for rubber, plastics, and resins. Krypton is a gas at room temperature. About Krypton. Similarly, number of neutrons in Kr-92 = 92 -36 = 56. Number of electrons 56. heart outlined. Number of protons, neutrons and electrons of chemical elements 02.12.2021 27.02.2022 support Proton (from Ancient Greek. The nucleus consists of 56 protons (red) and 82 neutrons (blue). Barium-132 is composed of 56 protons, 76 neutrons, and 56 electrons. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. The number of neutrons in the atoms of many elements is the same. There are 5 stable isotopes of Calcium (Ca) that are found in nature, and the number of neutrons in those range from 20 to 26. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Applications. 40 = 18+ N 40 = 18 + N. Ba Barium element 56 Appearance: Silvery gray Mass Number: 137 Atomic weight:137.327 g/mol Atomic number (Z): 56 Electrons: 56 Protons: 56 Neutrons: 81 The symbol given is N for Nitrogen. Barium occurs in 6 natural isotopes: 132 Ba, 134 Ba, 135 Ba, 136 Ba, 137 Ba and 138 Ba. The mass number is 235. Barium is a alkaline earth metal element. What is the function of the moderator in a nuclear reactor? What are the valencies of mercury? Neutron (n) = 14 7 = 7. Thanks 6. star. As an example, one of the fission products is 87 Br, containing too many neutrons and is consequently a emitter with a half-life of 55.7 s, decaying to 87 Kr. number of neutrons which are emitted in the process. atomic number 56 atomic weight 137.327 melting point 727 C (1,341 F) boiling point 1,805 C (3,281 F) specific gravity 3.51 (at 20 C, The nucleus consists of 56 protons (red) and 81 neutrons (orange). an element x a y emits 5 and 4 particles to give 8. How many neutrons does Barium-138 isotope have? Number of Neutrons is denoted by n0 symbol. Nitrogen atomic number and atomic weight. To use this online calculator for Number of Neutrons, enter Mass Number (A) & Atomic Number (Z) and hit the calculate button. The atomic number of barium represents the total number of electrons of barium. 1. What is the atomic number of potassium? Nitrogen atomic number and atomic weight. 2. How do you know the number of electrons? Answer: 82. add up the mass of protons and neutrons times electrons. Is Barium-137 isotope radioactive? Applying the formula above, the number of neutrons will be: # number of neutrons = Mass Number - Number of Protons. 100 = x +56 44 = x-Then, find the element in the periodic table that contains 44 protons. 6. Z, the atomic number, is simply the number of protons, fundamental, massive, positively charged particles, found within the nucleus. #2 Write Electron Configuration of Barium. CONCEPT:. Every atom is made of sub-atomic particles, including neutrons and protons. the number of the protons. An atom of Krypton has a full outer shell so it is inert. Molecular Weight. This nuclide decays by double electron capture (absorbing two electrons and emitting two neutrinos), with a half-life of Give the isotope symbol and number of neutrons in one atom of the following elements. add up the mass of protons and neutrons. # number of neutrons = 12. One atom of barium has 56 protons. It has the symbol Ba, and atomic number 56. Barium is the fifth element in group 2 and is a soft, silvery alkaline earth metal. a. number of protons _____ b.number of neutrons_____ c.number of electrons in a neutral atom_____ And the mass number is the number of protons plus the number of neutrons, Z is the atomic number, which we get right off the periodic table. What is atomic number for Barium-138 isotope? Barium is a chemical element with the symbol Ba and atomic number 56. Barium (Ba). answer choices. Barium, symbol Ba, has a Body Centered Cubic structure and Silver color. Of course, the nucleus could contain different numbers of neutrons, massive, neutrally charged particles, and this e.86 electrons,125 neutrons,82 protons (charged atom _____ f.0 neutrons _____ 11 .If you know only the following information can you always determine what the element is? it has the symbol Ba. Barium (Ba). The chemical symbol for Barium is Ba. grendeldekt and 8 more users found this answer helpful. Atomic Weight. So from here we can find the number of protons, number of Protons will be equal to a minus number of neutrons number of neutrons. A: Given : number of protons = 2 number of neutrons = 1 number of electrons = 1 Q: Give the number of protons and neutrons in the nucleus of boron-11 A: The number of protons and neutrons in the nucleus of boron-11 is to be determined. Since argon argon 's atomic number is 18 18, Ar Ar has 18 18 protons. This isotope is cation with oxidation number +2, because barium has two protons more than electrons (56 - 54 = 2). And the 137 for the mass number designates 137 total protons protons plus neutrons. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Barium is 56. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z 1) negative electrons in the atom. Answer: 56. back to Barium isotopes list. Number of protons 56. Element Element Symbol Z A Protons Neutrons Electrons Nitrogen N 7 15 7 8 7 Calcium 42 14 16 Lets do the first row together. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal.Because of its high chemical reactivity, barium is never found in nature as a free element.. Ba. How many protons does Barium-137 isotope have? Enter your answers in order given in the table. What is atomic number for Barium-137 isotope? the sum of the number of protons, neutrons, and electrons. 56. Since the vast majority of an atoms mass is found its protons and neutrons, subtracting the number of protons (i.e. 10.) Krypton has 36 protons and 48 neutrons in its nucleus giving it an Atomic Number of 36 and an atomic mass of 84. Atomic Mass = # of --- : number of protons plus neutrons. It has a role as a cofactor. Comparing the Barium of 56 protons with Xenon of 54 protons (even number ) we conclude that the structure of Barium has the same high symmetry as that of Xenon. Atomic number is equal to the number of protons in an atom-fill in the number of protons = 7. the sum of the number of the neutrons and electrons. Is Barium-138 isotope stable? 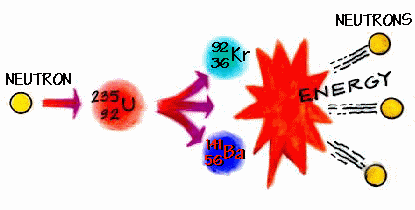 Barium (2+) is a barium cation, a divalent metal cation and a monoatomic dication. Fill in the known values where N N represents the number of neutrons. Naturally occurring barium (56 Ba) is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, identified as being unstable by geochemical means (from analysis of the presence of its daughter xenon-130 in rocks) in 2001. 727 C. Explanation: Element Barium. Based on the atomic number of the element, the mass number, and the number of neutrons, three things can be considered. Barium - Ba (EnvironmentalChemistry.com)- Comprehensive information for the element Barium - Ba is provided by this page including scores of properties, element names in many languages, Answer: No. For the second question, no, U 235 is not the only fissile nucleus. Request stable isotope quote. Number of neutrons (typical isotopes) 134-138. A single atom of barium has 56 protons in its nucelus and in its elemental form has 56 electrons in electron orbitals surrounding the nucelus. It is an Alkaline earth metal and is located in Group 2 of the periodic table. The chemical symbol for Barium is Ba. The nucleus consists of 56 protons (red) and 81 neutrons (orange). It is often used in barium-nickel alloys for spark plug wire. Atoms Strontium and barium have similar chemical properties because atoms of these elements have the same number of - 20728432 daniela2004alejandra daniela2004alejandra 01/19/2021 Strontium and barium have similar chemical properties because atoms of these elements have the same number of 1.protons 2.neutrons 3.electron Element 56 of Periodic table is Barium with atomic number 56, atomic weight 137.327. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. "first, Main) - Elementary particle, having an electric charge +1 e . So 56 protons is our atomic number Z.
Barium (2+) is a barium cation, a divalent metal cation and a monoatomic dication. Fill in the known values where N N represents the number of neutrons. Naturally occurring barium (56 Ba) is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, identified as being unstable by geochemical means (from analysis of the presence of its daughter xenon-130 in rocks) in 2001. 727 C. Explanation: Element Barium. Based on the atomic number of the element, the mass number, and the number of neutrons, three things can be considered. Barium - Ba (EnvironmentalChemistry.com)- Comprehensive information for the element Barium - Ba is provided by this page including scores of properties, element names in many languages, Answer: No. For the second question, no, U 235 is not the only fissile nucleus. Request stable isotope quote. Number of neutrons (typical isotopes) 134-138. A single atom of barium has 56 protons in its nucelus and in its elemental form has 56 electrons in electron orbitals surrounding the nucelus. It is an Alkaline earth metal and is located in Group 2 of the periodic table. The chemical symbol for Barium is Ba. The nucleus consists of 56 protons (red) and 81 neutrons (orange). It is often used in barium-nickel alloys for spark plug wire. Atoms Strontium and barium have similar chemical properties because atoms of these elements have the same number of - 20728432 daniela2004alejandra daniela2004alejandra 01/19/2021 Strontium and barium have similar chemical properties because atoms of these elements have the same number of 1.protons 2.neutrons 3.electron Element 56 of Periodic table is Barium with atomic number 56, atomic weight 137.327. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. "first, Main) - Elementary particle, having an electric charge +1 e . So 56 protons is our atomic number Z. 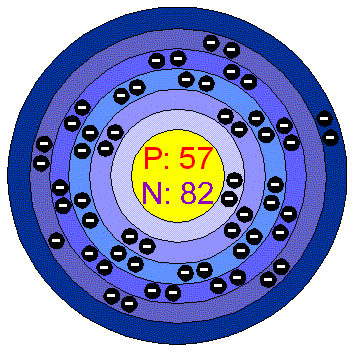 star. multiply electrons & protons. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. How to calculate Number of Neutrons using this online calculator? Share. Ba. The barium atom has a radius of 222 pm and a Van der Waals radius of 268 pm. Answer is: this is isotope of barium, because barium has atomic number 56 (number of protons), mass number of this isotope of barium is 130 (56 protons + 76 neutrons). Caption. Barium-135 is composed of 56 protons, 79 neutrons, and 56 electrons. Barium-136 is composed of 56 protons, 80 neutrons, and 56 electrons. Barium-137 is composed of 56 protons, 81 neutrons, and 56 electrons. How many neutrons does barium Ba have? To find the number of protons in argon argon, first locate the element on the periodic table. Modern Periodic Table.
star. multiply electrons & protons. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. How to calculate Number of Neutrons using this online calculator? Share. Ba. The barium atom has a radius of 222 pm and a Van der Waals radius of 268 pm. Answer is: this is isotope of barium, because barium has atomic number 56 (number of protons), mass number of this isotope of barium is 130 (56 protons + 76 neutrons). Caption. Barium-135 is composed of 56 protons, 79 neutrons, and 56 electrons. Barium-136 is composed of 56 protons, 80 neutrons, and 56 electrons. Barium-137 is composed of 56 protons, 81 neutrons, and 56 electrons. How many neutrons does barium Ba have? To find the number of protons in argon argon, first locate the element on the periodic table. Modern Periodic Table.  ( See my STRUCTURE OF Xe-124..Xe-134 ). Element Barium (Ba), Group 2, Atomic Number 56, s-block, Mass 137.327. Answer: 81. Chemistry questions and answers. 2005-06-24. 56 56 protons Fill in the known values where N N represents the number of neutrons. Therefore, the number of neutrons in nitrogen is 7. Strontium and barium have similar chemical properties because atoms of these elements have the same number of. Is Barium-138 isotope radioactive? 137.33. S-32 and S-32. For ""^235U metal, Z, the atomic number = 92. soon thereafter into a nucleus of barium (Ba) and a nucleus of krypton (Kr). SURVEY. atomic number 56 atomic weight 137.327 melting point 727 C (1,341 F) boiling point 1,805 C (3,281 F) specific gravity 3.51 (at 20 C, It is never found in nature in its pure form due to its reactivity with air. barium
( See my STRUCTURE OF Xe-124..Xe-134 ). Element Barium (Ba), Group 2, Atomic Number 56, s-block, Mass 137.327. Answer: 81. Chemistry questions and answers. 2005-06-24. 56 56 protons Fill in the known values where N N represents the number of neutrons. Therefore, the number of neutrons in nitrogen is 7. Strontium and barium have similar chemical properties because atoms of these elements have the same number of. Is Barium-138 isotope radioactive? 137.33. S-32 and S-32. For ""^235U metal, Z, the atomic number = 92. soon thereafter into a nucleus of barium (Ba) and a nucleus of krypton (Kr). SURVEY. atomic number 56 atomic weight 137.327 melting point 727 C (1,341 F) boiling point 1,805 C (3,281 F) specific gravity 3.51 (at 20 C, It is never found in nature in its pure form due to its reactivity with air. barium